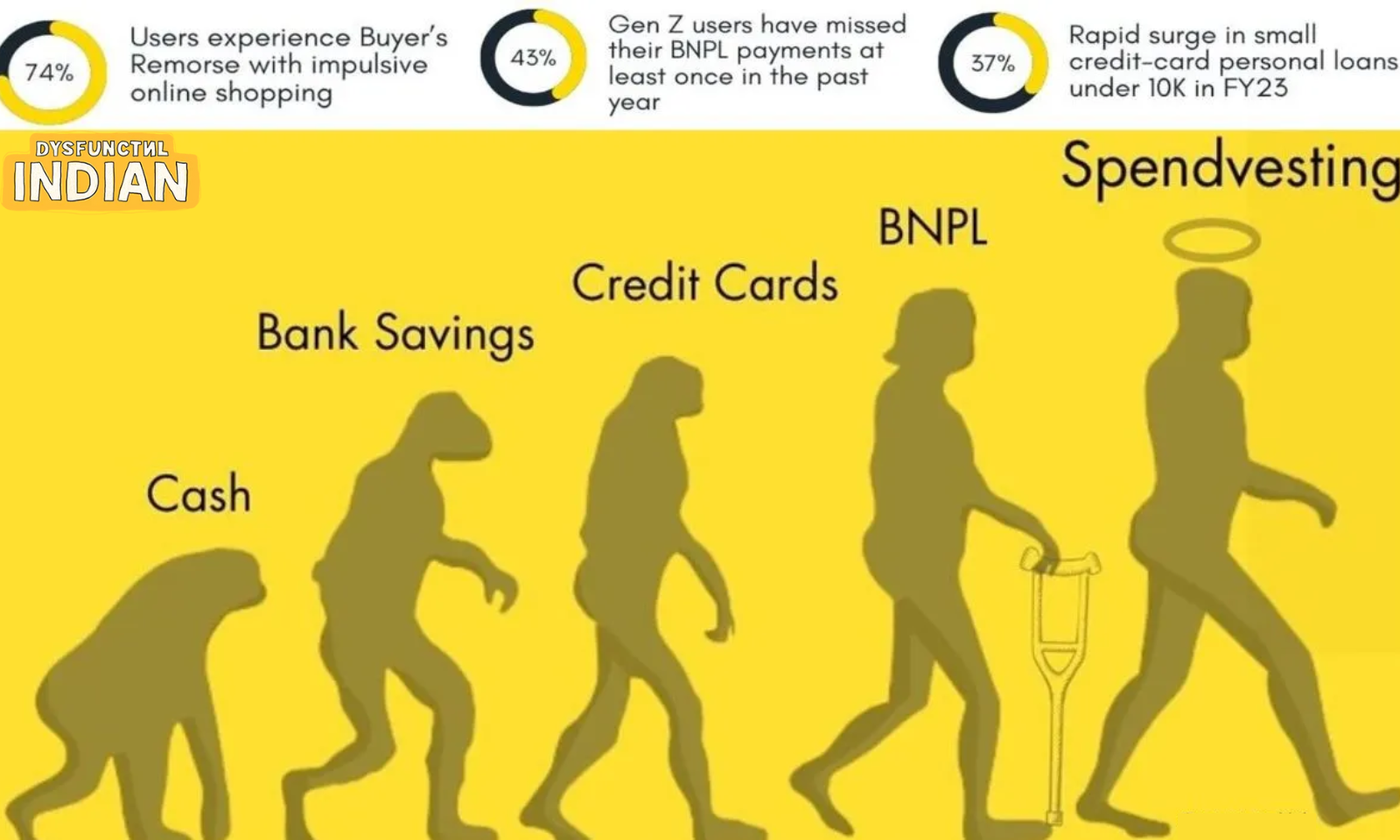
AstraZeneca Admits its Covid Vaccine Covishield Can Lead to Rare Adverse Reactions
Posted by admin on 2024-04-30 |
· The pharmaceutical company is being sued in a
class action over claims that its vaccine against Covid-19.
· AstraZeneca has admitted that Covishield can
"in very rare cases, cause TTS". TTS stands for Thrombosis with
Thrombocytopenia Syndrome.
· Covishield was developed by AstraZeneca and
was produced by the Serum Institute of India.
AstraZeneca, for the first time, admitted in
its court documents that its Covid vaccine can cause rare side effects. A
British-Swedish multinational pharmaceutical and biotechnology company,
AstraZeneca, has admitted that its COVID vaccine, Covishield, can cause TTS,
albeit rarely.
In India, it was one of the widely
administered COVID vaccine.
What
is TTS?
Thrombosis with thrombocytopenia syndrome
(TTS) is a rare but serious condition characterized by blood clot formation
(thrombosis) coupled with low platelet counts (thrombocytopenia).
Typically, TTS presents symptoms such as
severe headaches, abdominal pain, leg swelling, shortness of breath, and
neurological deficits. Diagnosis involves blood tests to assess platelet levels
and imaging studies to detect blood clots.
Treatment for TTS requires a
multidisciplinary approach, including hospitalization, anticoagulation therapy,
and supportive care. Healthcare providers closely monitor patients with TTS due
to the potential for severe complications, including organ damage and death.
Allegations
against Vaccine
51 cases have been lodged in the High Court,
with victims and grieving relatives seeking damages estimated to be worth up to
£100 million.
While AstraZeneca is contesting the
allegations, it admitted in a legal document submitted to the High Court in
February that its Covid vaccine "can, in very rare cases, cause TTS."
Association
with India
AstraZeneca, in partnership with The
University of Oxford, developed the AZD1222 vaccine following the coronavirus
outbreak in 2020. In India and other low-and-middle-income countries, it was
manufactured and supplied under the name “Covishield” by Serum Institute of
India (SII) through a licence from the university and the Swedish-British
drugmaker.
According to the World Health Organization
(WHO), the vaccine is "safe and effective for all individuals aged 18 and
above," and the adverse effect prompting legal action is "very
rare."